
Ion Mobility of Proteins in Nitrogen Gas: Effects of Charge State, Charge Distribution, and Structure. Daniele Canzani, Kenneth J. Laszlo, Matthew F. Bush. J. Phys. Chem. A 2018, in press. (Link)
Ion mobility is emerging as a rapid and sensitive tool for structural characterization. Collision cross-section (Ω) values determined using ion mobility are often compared to values calculated for candidate structures generated through molecular modeling. Several methods exist for calculating Ω values, but the trajectory method explicitly includes contributions from long-range, ion–neutral interactions. Recent implementations of the trajectory method have significantly reduced its expense and have made applications to proteins far more tractable. Here, we use ion mobility experiments and trajectory method calculations to characterize the effects of charge state, charge distribution, and structure on the ion mobility of proteins in nitrogen gas. These results show that ion-induced dipole interactions Continue reading “New Publication: Ion Mobility of Proteins in Nitrogen Gas: Effects of Charge State, Charge Distribution, and Structure”
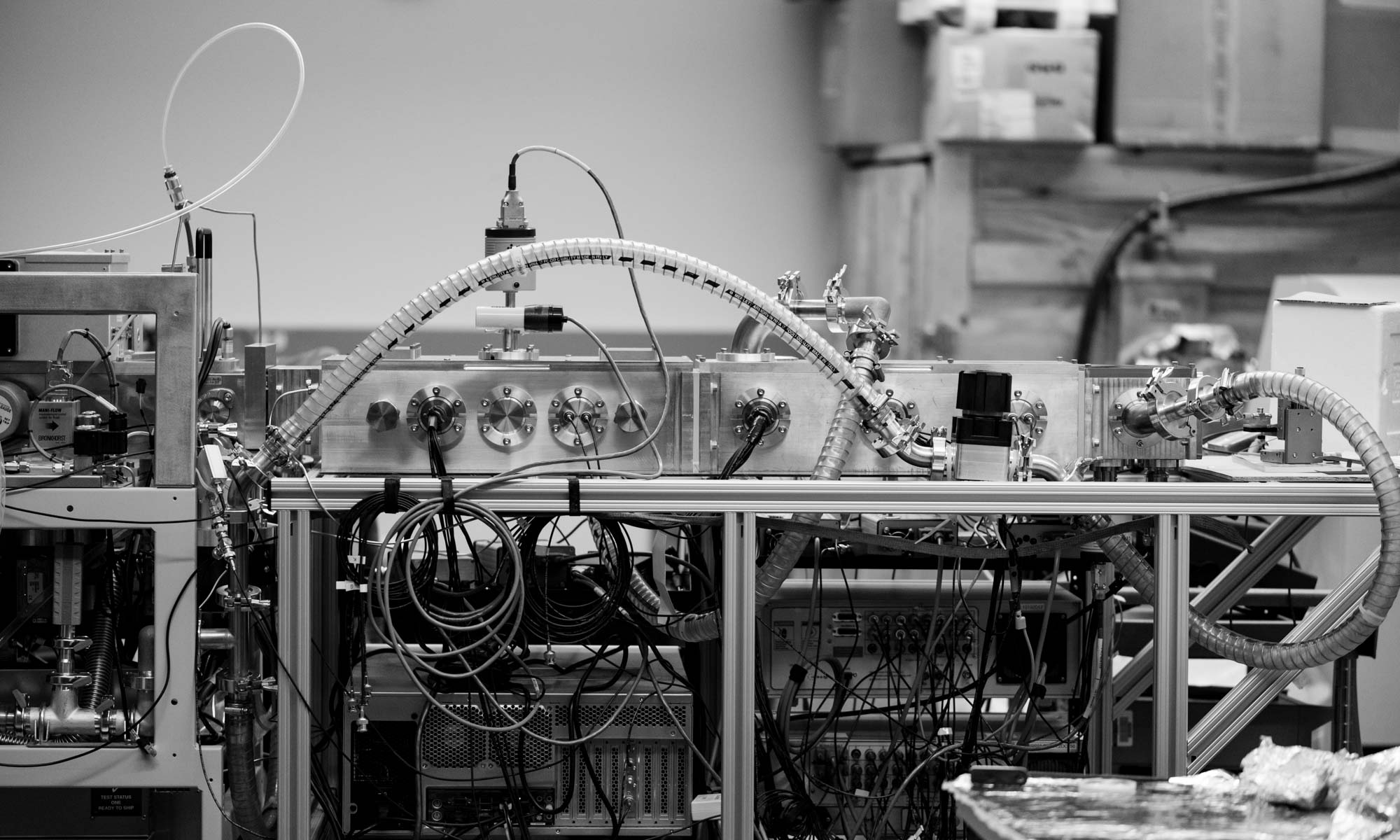

 Nonspecific Aggregation in Native Electrokinetic Nanoelectrospray Ionization. Kimberly L. Davidson; Derek R. Oberreit; Christopher J. Hogan; Matthew F. Bush. Int. J. Mass Spectrom. 2016, DOI: 10.1016/j.ijms.2016.09.013. (
Nonspecific Aggregation in Native Electrokinetic Nanoelectrospray Ionization. Kimberly L. Davidson; Derek R. Oberreit; Christopher J. Hogan; Matthew F. Bush. Int. J. Mass Spectrom. 2016, DOI: 10.1016/j.ijms.2016.09.013. ( Radio-Frequency (rf) Confinement in Ion Mobility Spectrometry: Apparent Mobilities and Effective Temperatures
Radio-Frequency (rf) Confinement in Ion Mobility Spectrometry: Apparent Mobilities and Effective Temperatures
 Analysis of Native-Like Proteins and Protein Complexes Using Cation to Anion Proton Transfer Reactions (CAPTR). Kenneth J. Laszlo; Matthew F. Bush. J. Am. Soc. Mass Spectrom. 2015, in press. (
Analysis of Native-Like Proteins and Protein Complexes Using Cation to Anion Proton Transfer Reactions (CAPTR). Kenneth J. Laszlo; Matthew F. Bush. J. Am. Soc. Mass Spectrom. 2015, in press. ( Collision cross section calibrants for negative ion mode traveling wave ion mobility-mass spectrometry. Jay G. Forsythe, Anton S. Petrov, Chelsea A. Walker, Samuel J. Allen, Jarrod S. Pellissier, Matthew F. Bush, Nicholas V. Hud, Facundo M. Fernández. Analyst 2015, 140, 6853-6861. (
Collision cross section calibrants for negative ion mode traveling wave ion mobility-mass spectrometry. Jay G. Forsythe, Anton S. Petrov, Chelsea A. Walker, Samuel J. Allen, Jarrod S. Pellissier, Matthew F. Bush, Nicholas V. Hud, Facundo M. Fernández. Analyst 2015, 140, 6853-6861. (


You must be logged in to post a comment.