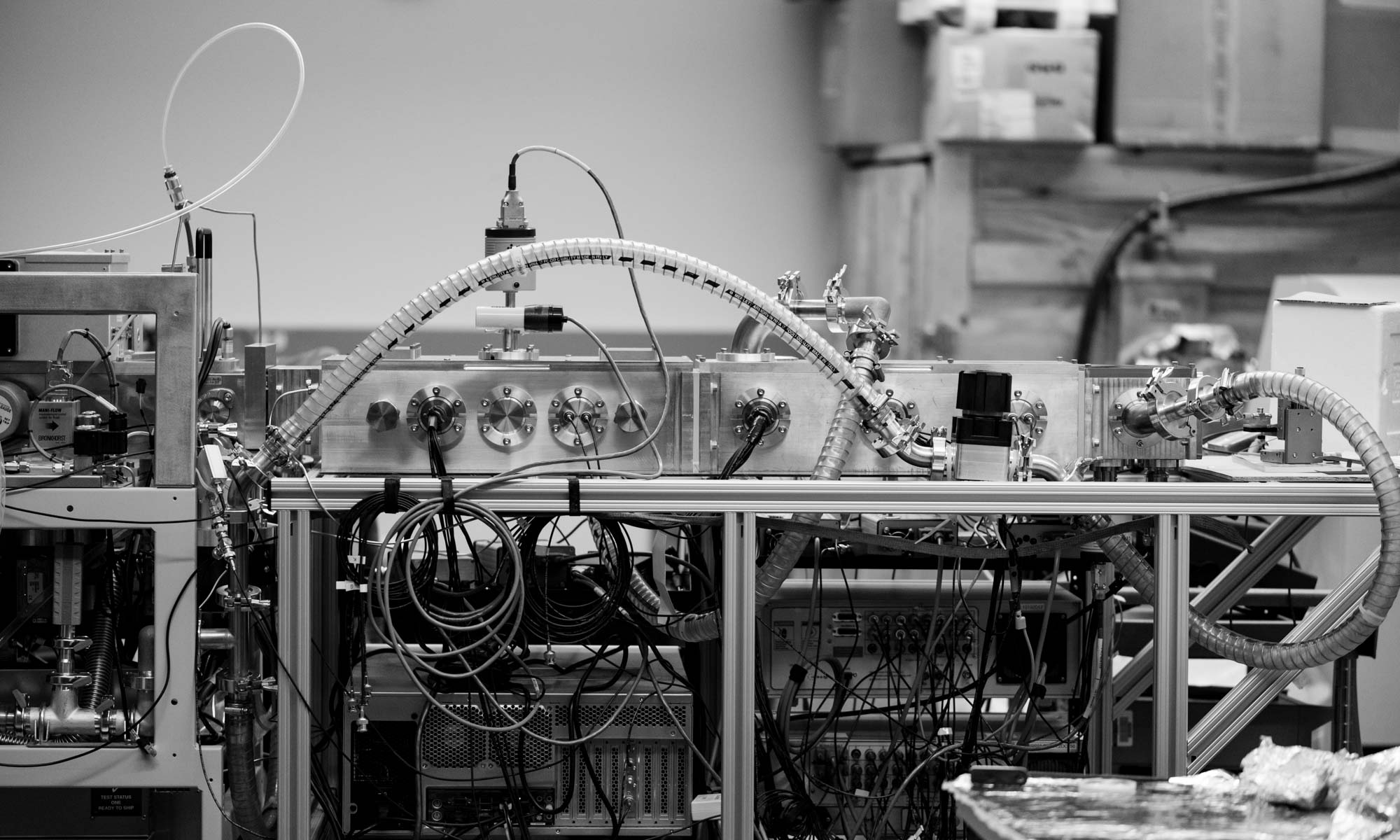
We are fabricating a new instrument for ion mobility mass spectrometry, which was designed by Sam Allen and Rae Eaton. Here is a video of some recent work by the machine shop in UW Department of Chemistry.
New Lab Member: Julia Greenwald
 The Bush Lab welcomes Julia Greenwald! Click here to learn more about Julia.
The Bush Lab welcomes Julia Greenwald! Click here to learn more about Julia.
New Publication: Nonspecific aggregation in native electrokinetic nanoelectrospray ionization
 Nonspecific Aggregation in Native Electrokinetic Nanoelectrospray Ionization. Kimberly L. Davidson; Derek R. Oberreit; Christopher J. Hogan; Matthew F. Bush. Int. J. Mass Spectrom. 2016, DOI: 10.1016/j.ijms.2016.09.013. (Link)
Nonspecific Aggregation in Native Electrokinetic Nanoelectrospray Ionization. Kimberly L. Davidson; Derek R. Oberreit; Christopher J. Hogan; Matthew F. Bush. Int. J. Mass Spectrom. 2016, DOI: 10.1016/j.ijms.2016.09.013. (Link)
Native mass spectrometry is widely used to determine the stoichiometries and binding constants of noncovalent interactions in solution. One challenge is that multiple analytes in a single electrospray droplet can aggregate during solvent evaporation, which will bias the distribution of oligomeric states observed during gas-phase measurements. Here, measurements of solution flow rates, electrospray currents, droplet size distributions, and nonspecific aggregation are used in conjunction with Poisson statistics to characterize the factors that control nonspecific aggregation during typical native mass spectrometry experiments. Continue reading “New Publication: Nonspecific aggregation in native electrokinetic nanoelectrospray ionization”
New Publication: Analysis of Native-Like Ions Using Structures for Lossless Ion Manipulations
 Analysis of Native-Like Ions using Structures for Lossless Ion Manipulations.
Analysis of Native-Like Ions using Structures for Lossless Ion Manipulations.
Samuel J. Allen, Rachel M. Eaton, and Matthew F. Bush.
Anal. Chem. 2016, DOI: 10.1021/acs.analchem.6b02089. (Link)
Ion mobility separation of native-like protein and protein complex ions expands the structural information available through native mass spectrometry analysis. Here, we implement Structures for Lossless Ion Manipulations (SLIM) for the analysis of native-like ions. SLIM has been shown previously to operate with near lossless transmission of ions up to 3000 Da in mass. Here for the first time, SLIM was used to separate native-like protein and protein complex ions ranging in mass from 12 to 145 kDa. The resulting arrival-time distributions were monomodal and were used to determine collision cross section values that are within 3% of those determined from radio-frequency-confining drift cell measurements. These results are consistent with the retention of native-like ion structures throughout these experiments. Continue reading “New Publication: Analysis of Native-Like Ions Using Structures for Lossless Ion Manipulations”
New Publication: Radio-Frequency (rf) Confinement in Ion Mobility Spectrometry
 Radio-Frequency (rf) Confinement in Ion Mobility Spectrometry: Apparent Mobilities and Effective Temperatures
Radio-Frequency (rf) Confinement in Ion Mobility Spectrometry: Apparent Mobilities and Effective Temperatures
Samuel J. Allen, Matthew F. Bush
J. Am. Soc. Mass Spectrom. 2016, DOI: 10.1007/s13361-016-1479-9. (Link)
Ion mobility is a powerful tool for separating and characterizing the structures of ions. Here, a radio-frequency (rf) confining drift cell is used to evaluate the drift times of ions over a broad range of drift field strengths (E/P, V cm–1 Torr–1). The presence of rf potentials radially confines ions and results in excellent ion transmission at low E/P (less than 1 V cm–1 Torr–1), thereby reducing the dependence of ion transmission on the applied drift voltage. Non-linear responses between drift time and reciprocal drift voltages are observed for extremely low E/P and high rf amplitudes. Under these conditions, pseudopotential wells generated by the rf potentials dampen the mobility of ions. The effective potential approximation Continue reading “New Publication: Radio-Frequency (rf) Confinement in Ion Mobility Spectrometry”
New Publication: Folding of Protein Ions in the Gas Phase after Cation-to-Anion Proton-Transfer Reactions (CAPTR)

Folding of Protein Ions in the Gas Phase after Cation-to-Anion Proton-Transfer Reactions (CAPTR)
Kenneth J Laszlo, Eleanor B. Munger, and Matthew F Bush
J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b04282. (Link)
The structure and folding of a protein in solution depends on noncovalent interactions within the protein and those with surrounding ions and molecules. Decoupling these interactions in solution is challenging, which has hindered the development of accurate physics-based models for structure prediction. Investigations of proteins in the gas phase can be used to selectively decouple factors affecting the structures of proteins. Here, we use Cation to Anion Proton Transfer Reactions (CAPTR) to reduce the charge states of denatured ubiquitin ions in the gas phase, and ion mobility to probe their structures. Continue reading “New Publication: Folding of Protein Ions in the Gas Phase after Cation-to-Anion Proton-Transfer Reactions (CAPTR)”
New Lab Member: Anna Bakhtina
 The Bush Lab welcomes Anna Bakhtina, who is an undergraduate biochemistry major. Click here to learn more about Anna.
The Bush Lab welcomes Anna Bakhtina, who is an undergraduate biochemistry major. Click here to learn more about Anna.
New Lab Member: Jack Buckner
 The Bush Lab welcomes Jack Buckner, who is visiting this summer from Carleton College. Click here to learn more about Jack.
The Bush Lab welcomes Jack Buckner, who is visiting this summer from Carleton College. Click here to learn more about Jack.
Bush Lab at ASMS 2016
The Bush Lab and collaborators will present the following talks and posters at American Society for Mass Spectrometry Annual Conference in San Antonio, TX (June 5-9):
- Evaluating Gas-Phase Folding of Protein Ions Using Cation to Anion Proton Transfer. Kenneth J. Laszlo; Eleanor B. Munger; Stephanie C. Heard; Matthew F Bush. (MOB 8:50)
- Amino Acid Separation using Different Drift Gases in an RF-Confining Drift Cell. Kimberly Davidson; Matthew F Bush (MOC 9:30)
- Analysis of Native-Like Protein and Protein Complex Ions using Structures for Lossless Ion Manipulations (SLIM). Samuel J. Allen; Rachel M. Eaton; Matthew F. Bush (TP 448)
Sam Allen and Matt Bush will also participate in the Fundamentals Interest Group workshop titled “Modification of Commercial Instruments for Fundamental Research”, which will be held in Room 302A on Tuesday, June 7.
We look forward to seeing everyone in San Antonio!
2016 Chemistry Awards Dinner

Congratulations to (from left):
- Sam Allen, who received a graduate student fellowship from the Irving and Mildred Shain Endowed Fund in Chemistry
- Kim Davidson, who received a graduate student fellowship from the Reinhardt Family Endowed Fund in Chemistry
- Rae Eaton, who received a Pacific Northwest National Laboratory Graduate Fellowship and a National Science Foundation Graduate Research Fellowship
- Cece Hong, who received a graduate student fellowship from the Schomaker Endowed Fund in Chemistry


You must be logged in to post a comment.